The Rise of Chemistry: From Alchemy to Modern Science
Written on
Chapter 1: The Origins of Chemistry
In this exploration, we revisit the dawn of chemistry through significant experiments involving "air." A heartfelt thank you to those who've stayed engaged during the hiatus.
To kick things off, it’s vital to recognize that the term chemistry was not in use during the era typically marked as its inception. In other words, the individuals conducting what we now refer to as chemistry were unaware that they were engaged in this scientific practice. The word "science" itself is a relatively recent concept; a more fitting description for their work would be a “collection of studies.” The early studies that later defined chemistry primarily focused on "elements," or fundamental substances that could not be broken down further (pure substances). Aristotle introduced what could be dubbed the "Captain Planet elements": earth, water, air, and fire. This concept, endorsed by Aristotle, persisted for centuries.
With this foundational theory in place, progress in chemical understanding was slow. However, a group emerged that prioritized the hands-on manipulation of these elements, becoming known as alchemists. Often dismissed as eccentric due to their cryptic writings, recent analysis has revealed a deeper significance to their work, which was often couched in symbolism—likely as a means to safeguard their secrets. Alchemists were adept practitioners of empirical chemistry, and while they delved into philosophical musings (like the quest for the Philosopher’s Stone), their contributions laid the groundwork for what would evolve into chemistry. Through their pursuits of transforming substances into gold, they gained invaluable insights into the chemical and physical properties of elements.
As medieval times approached, scholars began to pose more fundamental inquiries about natural phenomena, such as, “What occurs when I burn this substance?” The practice of burning materials became a favored method of analysis, akin to how modern organic chemists use NMR spectroscopy today. Unfortunately, the opportunity to explore these questions was limited to the affluent. Enter Robert Boyle, a nobleman who published a comprehensive work that sought to merge natural philosophy (theory) with the practical findings of alchemists. This publication is often seen as a pivotal moment in the legitimization of chemistry as a formal science—it even marked the first use of the term chemistry in a context related to the contemporary field (spelled "chymistry").
Despite Boyle's efforts, chemistry remained largely obscure for another century. During this period, the term "phlogiston" emerged to describe the fire element and became a significant hurdle for future chemists. Unfortunately, the early chemists mistakenly chose to study one of the most challenging elements—oxygen—a colorless, tasteless, and odorless gas that was referred to simply as "air" at the time, in keeping with the longstanding elemental theory. This brings us to Joseph Priestley, who, through various experiments involving mercury, was the first to identify oxygen, then termed "dephlogisticated air," due to its ability to support combustion. A quick note: combustion was viewed as the transfer of phlogiston from one substance to another; since oxygen facilitated fire, it was considered "dephlogisticated."
Now that we are up to speed, let’s introduce our next key figure: Joseph Black.

Joseph Black, initially training to be a doctor, became captivated by the remedies for indigestion. Given the trend of analyzing the byproducts of burning or heating substances, it was logical for someone with his interests to experiment with heating chalk (calcium carbonate). His enthusiasm for the study of elements eventually led him to abandon his medical career altogether. His experiments culminated in the discovery of carbon dioxide—not an element but a notable achievement, given its own colorless and odorless nature. He referred to it as "fixed air" due to its inability to support combustion—it had become “fixed” with phlogiston. Black also demonstrated that carbon dioxide was present in human breath and as a component of regular air.
Next in line is Henry Cavendish, a name likely familiar to chemistry enthusiasts. To cut to the chase: he discovered hydrogen. His analytical approach diverged slightly; he mixed substances with acids, generating a gas, which he dubbed "inflammable air," highlighting its flammability (the term 'inflammable' can be misleading, suggesting non-flammability).
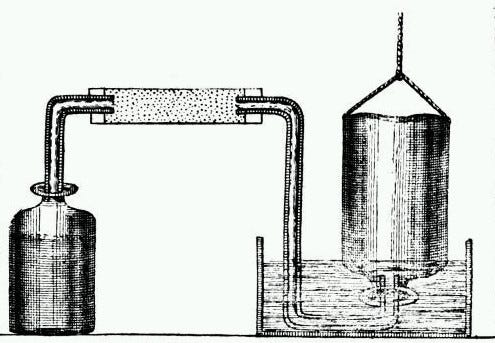
At this point, it’s worth discussing how hydrogen fit into the phlogiston theory of combustion. According to Cavendish's logic, metals (not yet recognized as pure substances) contained both phlogiston and calx (an old term for metal oxide). When metals were combined with acid and air, they released phlogiston into the air, resulting in a dissolved calx.
metal (? + calx) + acid + air → dissolved calx + acid + inflammable air (air + ?)
Cavendish's work significantly reinforced the phlogiston theory of combustion, embedding it deeply in the minds of emerging chemists. Correcting this misconception would take approximately a century and hinder chemistry's advancement as a legitimate science.
Both Black's and Cavendish's groundbreaking experiments illuminated the field of chemistry, challenging the long-standing elemental theory. They revealed that there were indeed three distinct types of air, placing the ancient notion of air as a singular element in jeopardy.
Be forewarned: if you choose to delve into Cavendish’s original writings, you are stepping into a realm of scientific inquiry that may change your perspective forever.
If you've clicked that link, you have my admiration. However, your partner might not feel the same!
Cavendish also noted something intriguing but did not pursue it further: the condensation of droplets on his reaction vessel after combining inflammable air with dephlogisticated air. Modern scientists understand that this reaction between hydrogen (inflammable air) and oxygen (dephlogisticated air) produces water. This fundamental reaction would not only lay the groundwork for atomic theory but also ignite debates for centuries to come—and the clues were right there!
Ultimately, these experiments set the stage in Europe, fostering a growing interest in chemistry, but the field still required a unifying figure to consolidate its fragmented theories. That individual would come to be known as the Elon Musk of chemistry.
Works Consulted:
Brock, William H. The Chemical Tree: A History of Chemistry. New York: Norton and Co, 2000.
Ihde, Aaron J. The Development of Modern Chemistry. New York: Dover Publications, 1984.